TREATING PLASMINOGEN
DEFICIENCY TYPE 1 (PLGD-1)
Available treatments
Plasminogen replacement therapy, derived from human plasma, is administered intravenously and temporarily restores plasminogen to therapeutic levels. This increase of plasminogen also increases activity levels, allowing the body to properly break down fibrin as long as plasminogen levels are consistently maintained. This systemic therapy was approved by the FDA in 2021 and can be administered at home by the patient or a caregiver.1
Fresh frozen plasma (FFP) has been used as a source of plasminogen, administered either intravenously or as eye drops for ligneous conjunctivitis. Because plasminogen is present in such low amounts in plasma, infusing enough to sufficiently raise systemic plasminogen levels can result in fluid overload. This treatment method is not FDA approved.2
Prior to the approval of plasminogen replacement therapy, removal or surgical excision of lesions was the primary treatment approach. While it can be successful initially, removal or surgery often triggers accelerated lesion regrowth if not followed up by treatment with plasminogen replacement therapy.2
Local or systemic use of high-dose corticosteroids, immunosuppressants, proteolytic enzymes, anti-inflammatory, antibiotic or anticoagulant agents have been met with limited or no success in clearing lesions and are not approved by the FDA to treat PLGD-1.2,3
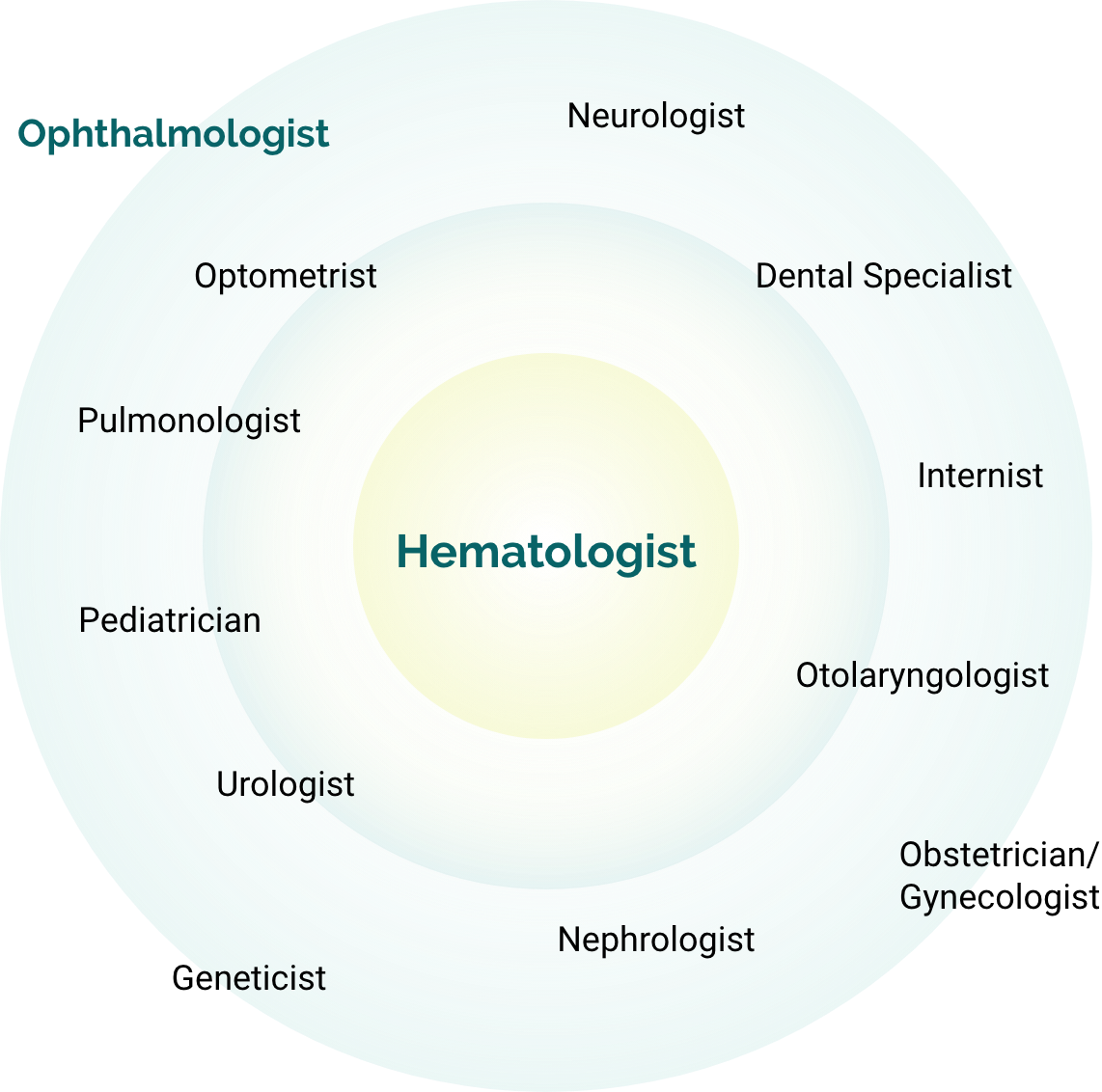
Specialists managing PLGD-14
Ophthalmologists have the unique opportunity to recognize what others have overlooked.2
Patients often turn to ophthalmologists first, though an array of specialists can serve as initial points of care depending on the patient's case, age, and lesion location.2
Caring for people with PLGD-1 will likely require coordinated care from healthcare providers across multiple disciplines, with a hematologist serving as the central point of care.3
Untreated lesions can have serious consequences4:
- Blindness
- Tooth loss
- Compromised organ function
- Infertility
- Reduced quality of life
- Life-threatening airway complications
- Periodontal destruction
- Organ system failure
- Hearing loss
See available resources
for expert insights, patient information, support tools, and more.
VIEW RESOURCES >References: 1. RYPLAZIM [prescribing information]. Kedrion Biopharma Inc. 2024. 2. Congenital type 1 plasminogen deficiency. NORD. Updated February 6, 2025. Accessed December 4, 2025. https://rarediseases.org/rare-diseases/congenital-plasminogen-deficiency 3. Shapiro AD, Nakar C. How I treat type 1 plasminogen deficiency. Blood. 2025;145(25):2954-2965. 4. Schuster V, Hügle B, Tefs K. Plasminogen deficiency. J Thromb Haemost. 2007;5(12):2315-2322. 5. Shapiro AD, Menegatti M, Palla R, et al. An international registry of patients with plasminogen deficiency (HISTORY). Haematologica. 2020;105(3):554-561.
